- HOME
- Custom Synthesis, Peptide Drug (GMP)
- Custom Peptide Synthesis
- Peptide Analogs for Drug Discovery
Peptide Analogs for Drug Discovery
Synthesis of Side Products For Peptide Drug Development
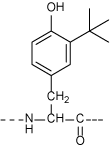
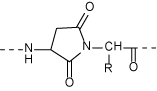
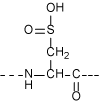
Due to the intrinsic property of the peptide, it is a prerequisite in terms of quality control and safety to recognize the small amount of contaminants in the peptidic medicine. Idendification is readily achieved utilizing the synthetic replica of such contaminants as authentic samples. Chemical synthesis of the site-specifically modified peptide is often more difficult than that of the intact molecule. However, we offer any such compounds upon request by applying our vast knowledge of peptide synthesis and the general organic chemistry. Some of the modifications to consider from the structure of the peptide are shown below, all of which are the target of our synthetic chemists!
For example
| Asp-X, Asn-X |
|
|---|---|
| Oxdation of Cys |
|
| Oxdation of Met |
|
| N-terminal Gln, Glu |
|
| Hydrolysis |
|
| Alkylation |
|
| Acylation |
|
| Others |
|
Succinimide
Sulfinic Acid
Tyr(3-But)